Seminar: Organic, supramolecular, medicinal chemistry
| October 11 | 11.00 a.m.-12.00 p.m. | Room 2.3.13
Mauro Mocerino, Professor, Curtin University, Perth, Australia

Synthesis and application of calixarenes in supramolecular and medicinal chemistry
Host: Paula M. Marcos
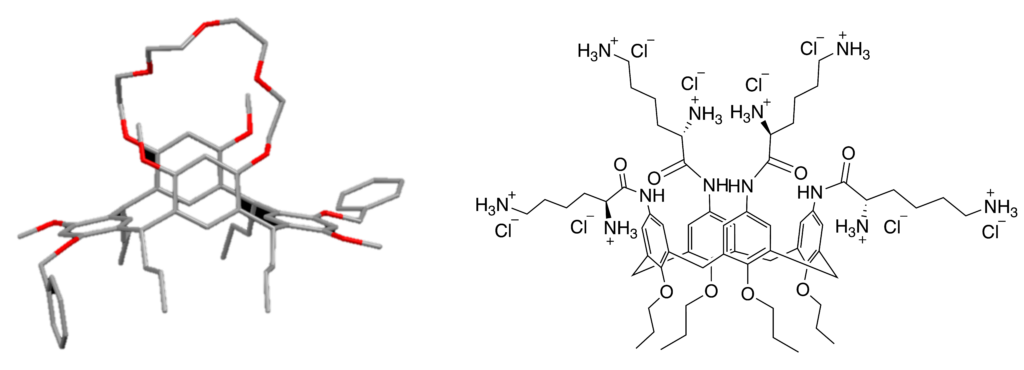
Calixarenes and resorcinarenes are cyclophanes in which the wider rim may be selectively and variously functionalised to enable them to engage in a range of non-covalent interactions. This seminar will present three projects relating to the chemistry of calixarene and resorcinarene derivatives. Many calixarene derivatives have shown gelation properties, and one such example is the proline funtionalised calix[4]arene which was found to be an anion triggered hydrogelator.1,2 C4 symmetric tetramethoxyresorcinarenes can be easily synthesized in a single step in high yields.3,4 These have a chiral cavity which could be used to selectively bind and separate enantiomers of racemic mixtures of pharmaceutically-relevant chiral molecules. Functionalising the resorcinarene with a bridge that spans across the wider rim (see figure below) should enhance encapsulation of guest species.5 Calix[4]arenes have also been shown to inhibit the protein-protein interactions of the L1 proteins of human papillomavirus (HPV).6-8 These calix[4]arenes were either anionic or zwitterionic at physiological pH. To investigate whether cationic analogues would have a similar or improved effect, a series of calix[4]arene derivatives functionalized with groups that would render them positively charged at physiological pH were synthesized (see an example below). These were then evaluated for their biological activity against HPV and the protozoans T.b.rhodesiense and P.falciparum. the causative agents for Human African Trypanosomiasis (HAT) and malaria respectively.
1. Becker, T., Goh, C. Y., Jones, F., McIldowie, M. J., Mocerino, M., Ogden, M. I. Chemical Communications, 2008, 3900.
2. Goh, C. Y., Becker, T., Brown, D. H., Skelton, B. W., Jones, F., Mocerino, M., Ogden, M. I. Chemical Communications, 2011, 47, 6057.
3. McIldowie, M. J.; Mocerino, M.; Skelton, B. W.; White, A. H. Organic letters 2000, 2, 3869.
4. McIldowie, M. J.; Mocerino, M.; Ogden, M. I. Supramolecular Chemistry 2010, 22, 13.
5. Tan, D. A., Massera, C., McIldowie, M. J. and Mocerino, M., Eur. J. Org. Chem. 2020, 5695
6. Zheng, D.-D., Fu, D.-Y., Wu, Y., Sun, Y.-L., Tan, L.-L., Zhou, T., Ma, S.-Q., Zhe, X., Yang, Y.-W., Chemical Communications, 2014; 50, 3201.
7. Fu, D.-Y., Lu, T., Liu, Y.-X., Li, F., Ogden, M. I., Wang, Y., Wu, Y., Mocerino, M., ChemistrySelect, 2016, 1, 6243.
8. Goh, C.-Y., Fu, D.-Y., Duncan, C. L., Tinker, A., Li F., Mocerino, M., Ogden, M. I., Wu, Y., Supramolecular Chemistry, 2020, 32, 345.